
periodic table pdf with electron configurations pdf
The periodic table organizes elements based on atomic structure, while electron configurations reveal the distribution of electrons in an atom, essential for understanding chemical properties and behavior.
1.1 Overview of the Periodic Table
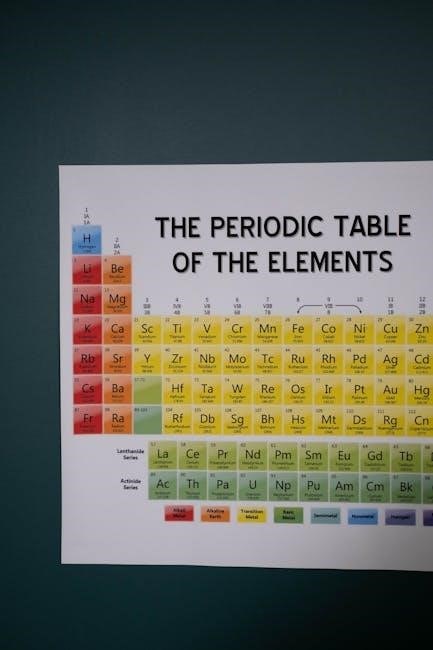
The periodic table is a tabular arrangement of chemical elements, organized by atomic number, electron configurations, and recurring chemical properties. It includes essential data such as element symbols, names, atomic masses, and electron configurations, providing a structured framework for understanding the relationships and properties of all known elements in a clear and accessible format.
1.2 Importance of Electron Configurations
Electron configurations are crucial for understanding an element’s chemical behavior, as they determine its valence electrons, oxidation states, and bonding capabilities. This information is vital for predicting chemical reactions and properties, making electron configurations essential for both academic study and practical applications in chemistry and related fields, providing a foundation for understanding the periodic relationships of elements.

Structure of the Periodic Table
The periodic table is structured to organize elements by atomic number, displaying their chemical properties and electron configurations in a logical, visually accessible format for easy reference.
2.1 Layout and Organization
The periodic table is arranged in a grid with rows called periods and columns called groups or families. Elements are organized by increasing atomic number, showcasing periodic trends. Each cell contains essential data, including atomic number, symbol, name, and atomic mass. Electron configurations are often included, providing insight into chemical properties and behavior. This systematic layout facilitates easy identification of relationships and trends among elements.
2.2 Electron Configuration Notation
Electron configuration notation represents the distribution of electrons in an atom’s orbitals. It follows the Aufbau principle, using numbers and letters to denote energy levels and sublevels. For example, 1s²2p⁵ indicates two electrons in the 1s orbital and five in the 2p orbital. This notation is often simplified using noble gas notation, such as [Ne]3s²3p⁵ for chlorine, enhancing readability and conciseness in periodic table PDFs.
2.3 Relationship Between Electron Configurations and Chemical Properties
The arrangement of electrons in an atom determines its chemical properties. Elements with similar electron configurations exhibit analogous behaviors, such as halogens needing one electron to achieve noble gas stability. Periodic table PDFs highlight these patterns, making it easier to predict properties like valence, electronegativity, and reactivity based on electron configurations.

Key Features of the Periodic Table PDF
The periodic table PDF provides essential details such as atomic number, symbol, name, atomic mass, electron configurations, oxidation states, electronegativity, and phase information, making it a comprehensive resource.
3.1 Atomic Number, Symbol, and Name
Each element in the periodic table is uniquely identified by its atomic number, symbol, and name. The atomic number represents the number of protons, while the symbol is a shorthand notation. For example, hydrogen is represented by atomic number 1 and symbol H. These identifiers are essential for understanding and organizing elements within the periodic table PDF.
3.2 Atomic Mass and Oxidation States
The periodic table PDF includes atomic mass, providing the average mass of an element’s isotopes. Oxidation states indicate the element’s common charges in compounds, such as chlorine’s -1. These details enhance understanding of elemental properties and chemical behavior, making the table a comprehensive resource for students and researchers.
3.3 Electronegativity and Phase Information
The periodic table PDF includes electronegativity values, measuring an element’s ability to attract electrons, and phase information, indicating whether elements are solid, liquid, or gas at standard conditions. These details provide insights into chemical behavior and physical properties, enhancing the understanding of elements’ interactions and bonding tendencies.

Electron Configuration Basics
Electron configurations describe the distribution of electrons in an atom, following the Aufbau principle. They determine chemical properties and bonding, forming the foundation of periodic table organization.
4.1 Noble Gas Notation
Noble gas notation simplifies electron configurations by using the nearest noble gas in brackets, representing filled electron shells. This condensed form highlights valence electrons, making it easier to identify chemical trends and predict properties without writing out all inner electrons, enhancing clarity and efficiency in understanding periodic relationships.
4.2 Orbital Diagrams and Electron Filling Order
Orbital diagrams visually represent electron configurations, showing electrons in boxes with arrows indicating spin. The filling order follows the Aufbau principle, starting with lower energy levels and pairing electrons according to Hund’s rule. This systematic approach ensures accurate determination of electron arrangements, crucial for understanding chemical bonding and periodic trends in the periodic table PDF.

Historical Development of the Periodic Table
The periodic table’s development began with John Newlands’ Law of Octaves in 1864, followed by Dmitri Mendeleev’s breakthrough in 1869, laying the foundation for modern chemistry.
5.1 Early Contributions: John Newlands and the Law of Octaves
John Newlands proposed the Law of Octaves in 1863, arranging elements in octaves of eight, similar to music notes, observing recurring properties every eighth element. However, this theory had limitations, failing beyond calcium and not accounting for new discoveries, but it paved the way for Mendeleev’s periodic table.
5.2 Dmitri Mendeleev and the Modern Periodic Table
Dmitri Mendeleev developed the modern periodic table in 1869, arranging elements by atomic weight and recurring chemical properties. He predicted the existence of undiscovered elements to fill gaps, earning recognition for accurately forecasting properties of elements like gallium, germanium, and scandium. His work laid the foundation for understanding periodic trends and electron configurations.
Practical Applications of Electron Configurations
Electron configurations help determine chemical properties, predict valence electrons, and explain bonding behavior. They are essential for understanding periodic trends and chemical reactivity, aiding chemists in practical applications.
6.1 Determining Chemical Properties
Electron configurations reveal the arrangement of electrons in an atom, enabling the determination of chemical properties such as valence electrons and reactivity trends. By analyzing these configurations, chemists can predict how elements interact and form bonds. This understanding is crucial for identifying periodic trends, such as electronegativity and oxidation states, which are essential for predicting chemical behavior and reactions. The periodic table PDF simplifies this process by organizing elements with their electron configurations, making it a valuable tool for chemists and students alike.
6.2 Predicting Valence Electrons and Bonding
Electron configurations in the periodic table PDF allow chemists to identify valence electrons, which determine an element’s bonding capabilities. By examining the outermost electron shell, one can predict whether an element will form ionic or covalent bonds. This insight helps in understanding molecular structures and chemical reactions, making electron configurations a fundamental tool for predicting bonding behavior and molecular interactions accurately. The periodic table’s organization enhances this process, providing a quick reference for such analyses.
Benefits of Using a Periodic Table PDF
A periodic table PDF offers a comprehensive, easy-to-read layout of elements, including atomic data and electron configurations, making it a portable and accessible study tool.
7.1 Portability and Accessibility
A periodic table PDF is a portable and accessible tool, perfect for easy reference. It can be downloaded and printed, making it ideal for study or fieldwork. The PDF includes essential data such as atomic number, symbol, name, atomic mass, and electron configurations. Its digital format allows access on multiple devices without internet, enhancing its convenience and usability for learners and professionals alike.
7.2 Customization for Educational Purposes
Periodic table PDFs are highly customizable for educational needs. Educators can highlight specific elements, add notes, or focus on particular sections like electron configurations or oxidation states. This adaptability makes it a versatile teaching tool, allowing tailored learning experiences for students. The ability to modify content ensures relevance to course materials and enhances student engagement with complex chemistry concepts.

Advanced Topics in Electron Configurations
Explore exceptions to the Aufbau principle, such as chromium and copper, and delve into hybridization and molecular orbital theory, which refine electron configuration understanding and chemical bonding predictions.
8.1 Exceptions to the Aufbau Principle
Elements like chromium and copper deviate from the Aufbau principle, with electron configurations favoring stability over sequential filling. Chromium has [Ar] 3d⁵ 4s¹, and copper has [Ar] 3d¹⁰ 4s¹, due to exchange energy and orbital stability. These exceptions highlight the complexity of atomic structure and are clearly marked in periodic table PDFs for educational clarity and understanding of chemical behavior;
8.2 Hybridization and Molecular Orbital Theory
Hybridization explains the mixing of atomic orbitals to form molecular orbitals, enabling covalent bond formation. Molecular orbital theory describes how atomic orbitals combine to form bonding and antibonding orbitals, influencing molecular geometry and stability. These concepts complement electron configurations in understanding chemical bonding and are visually represented in periodic table PDFs for enhanced educational understanding of molecular structures and interactions.
The periodic table, with electron configurations, is central to understanding chemical properties and will continue to guide future scientific advancements and educational pursuits.
9.1 The Role of Electron Configurations in Modern Chemistry
Electron configurations are fundamental to modern chemistry, enabling the prediction of chemical properties, bonding patterns, and reactivity. They provide insights into valence electrons, oxidation states, and molecular interactions, making them essential for advancing materials science, drug design, and theoretical chemistry. Understanding electron configurations remains critical for innovation and education in the field.
9.2 Future Developments in Periodic Table Research
Future research may explore new elements, refine electron configuration theories, and integrate advanced computational methods. Discoveries in quantum mechanics and materials science could reveal novel properties, expanding the periodic table’s applications. Enhanced digital tools, like interactive PDFs, will make the table more accessible, fostering education and innovation in chemistry and related fields.